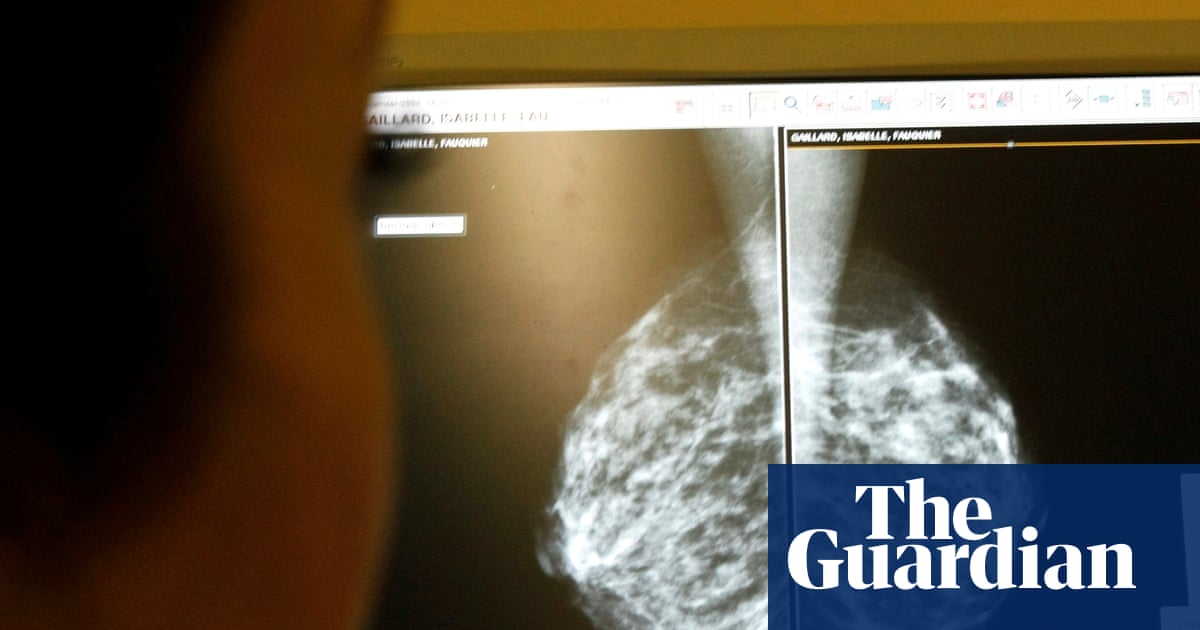
Campaigners have began a legal process intended to suspend a clinical trial of puberty blockers on the grounds that the research could prove harmful to the children taking part.
The study was commissioned in response to last year’s Cass review of gender identity services which found that gender medicine was an “area of remarkably weak evidence” and “built on shaky foundations”.
Originally used to treat early-onset puberty, puberty blockers had been prescribed off-label to children with gender dysphoria until the NHS banned their use last year after the Cass review.
Legal letters have been issued to the medical regulators responsible for the trial, and copied to the health secretary, Wes Streeting, and NHS England. The action has been launched by campaigners from the Bayswater Support Group, made up of parents of children and young adults who identify as trans or non-binary, along with the psychotherapist James Esses, who treats children with gender dysphoria, and Keira Bell, who began taking puberty blockers as a teenager before later detransitioning.
They say the trial “fails to safeguard the rights, safety and wellbeing of its subjects, who constitute highly vulnerable children” on the grounds that the treatment may irreversibly damage fertility. They argue that the research is “unlawful given the limited known benefits of treatment with puberty blockers”. Questioning the need to prescribe drugs, Esses said counselling has been shown to be effective for children with gender dysphoria.
Details of the NHS England-funded Pathways trial, which is scheduled to start in January, were revealed last month. Run by King’s College London researchers, the study will assess how to improve care for gender-questioning children and is expected to recruit about 226 young people over the next three years. The youngest participants could be 10 to 11 for biological girls and 11 to 12 for boys. The upper age limit for joining the study will be 15 years and 11 months.
One group will be given puberty blockers for two years, while the other will be given the drugs after a one-year delay. Researchers expect to publish results in around four years’ time.
Preliminary legal papers were issued to the Medicines and Healthcare products Regulatory Agency, which licensed the trial, and the Health Research Authority , whose role is to protect participants in medical trials. Both bodies said they were unable to comment on potential legal proceedings. The HRA said the trial had “all the necessary regulatory approvals that it needs to begin”.
Bell was the lead claimant in a 2020 judicial review of the now closed gender identity development service at the Tavistock Centre in London, questioning whether children under 16 are mature enough to give informed consent to be prescribed puberty blockers.
Bell said she believed the trial would harm children, and that her own experience of taking puberty blockers had left her extremely angry. “I didn’t know that I was essentially trapping my own mind from developing, because puberty doesn’t happen in a vacuum. It’s your whole body, it’s your brain sending signals to your body. So I didn’t understand any of that,” she told the BBC.
She also asked why the NHS was not conducting a study based on people who had already taken puberty blockers: “There are children who have already been down this pathway. I’m one of them. Why aren’t we doing follow-ups with people like me?”
Streeting has previously said the trial would “provide better evidence for how the NHS can support and treat young people with gender incongruence”. In a written statement to parliament in November he acknowledged it was a “challenging issue, where there are understandable concerns around safety, efficacy and consent”, but noted there were “strict eligibility criteria in place, including clinical review and parental consent”.
Jonathan Montgomery, a professor of health care law at University College London who advised the study team on how to ensure compliance with legal and ethical requirements, said the NHS’s new gender services had introduced better safeguarding and no longer assumed that puberty suppression was an appropriate option, offering instead a range of treatments.
“Young people and their families have been let down by a collective failure to generate the science that would enable them to take informed decisions,” he said. Baroness Cass recognised that research is needed to remedy that failure. Blocking all such research would compound these past wrongs.”

 1 month ago
36
1 month ago
36